Tim Friede and his team at the University Medical Centre Göttingen(UMG) lead Work Package 5 (WP5) – The Evaluation of the Effectiveness and Cost-effectiveness of the Intervention in close collaboration with the University Hospital of Cologne's health economics team. Tim is the Head of the Department of Medical Statistics at UMG, where he specialises in developing innovative clinical trial designs and analyses.
ESCAPE’s clinical trial is assessing the effect of ESCAPE’s blended-collaborative care (BCC) intervention on patient quality of life. The BCC model is supported by state-of-the-art algorithms and technologies and is delivered by care managers. For WP5, Tim and his team will analyse ESCAPE’s trial data to evaluate the efficacy of the BCC intervention. This includes analyses of outcomes such as health-related quality of life and clinical events (i.e., the number of patients admitted to hospitals or nursing homes), and safety aspects of the intervention.
When asked what Tim finds most innovative about ESCAPE, he quickly jumped into describing how the clinical trial was designed. A common criticism of randomised control trials (RCTs) is that the research populations are not representative of real-world patients. Clinical trials tend to attract certain types of patients, typically those who are more interested in healthcare and can navigate the healthcare system. Without a diverse patient population, bias is built into the RCT before it has even started. In ESCAPE’s project design phase of ESCAPE, Tim and Christoph Herrmann-Lingen (WP4 Leader) were discussing how to overcome this potential bias and decided to use a comprehensive cohort study design with an embedded randomised control trial. This is more complicated than a typical RCT, however, it works to overcome selection bias.
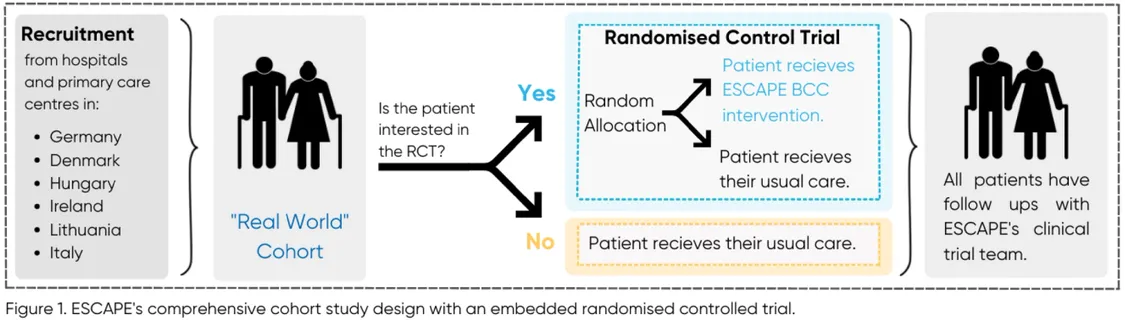
So, what is a comprehensive cohort study design with an embedded RCT? Essentially, patient recruitment happens in two phases. The first phase is for the cohort study (Figure 1 - “Real World” Cohort). Patients who agree to partake in the cohort study will have their quality of life monitored for up to three years. These patients will not receive any changes to their usual care, nor will they receive the BCC intervention.
In the second phase, the RCT is established. As the RCT is embedded within the cohort study, only those patients who agreed to the cohort study are invited to partake in the RCT. RCT patients will be randomly allocated into two groups, those who receive the BCC intervention and those who receive usual care (Figure 1 – Randomised Control Trial).
But how does this design make patient recruitment less biased? Because patients may be wary or less interested to participate in an experimental intervention, it can be difficult to recruit a diverse group for the RCT. However, patients may be comfortable having follow-ups to periodically monitor their usual care. This makes them perfect candidates for the “real-world” cohort. For those interested in the intervention, those patients can participate in the RCT. This two-phase system creates space for patients, who traditionally would not be interested in participating in a clinical trial, to still be represented via the cohort study. Thus, giving a more robust population to compare the BCC intervention to, and therefore increasing the validity of ESCAPE’s results.
ESCAPE’s trial is taking place in six different countries and languages and with a variety of illnesses. Statistically speaking, this diversity creates an abundance of data that Tim and the WP5 team are eager to explore. Tim poses that the benefit of EU wide projects is the built-in heterogeneity due to multiple countries, languages, cultures and healthcare systems. As such, replication, is to some extent, built into the design and ensures that ESCAPE’s BCC approach and ESCAPE’s results can be impactful on a European scale.
Tim’s team will continue running the analysis and monitor the trial’s data quality for the duration of the ESCAPE project. Keep an eye on the website for future updates.
