
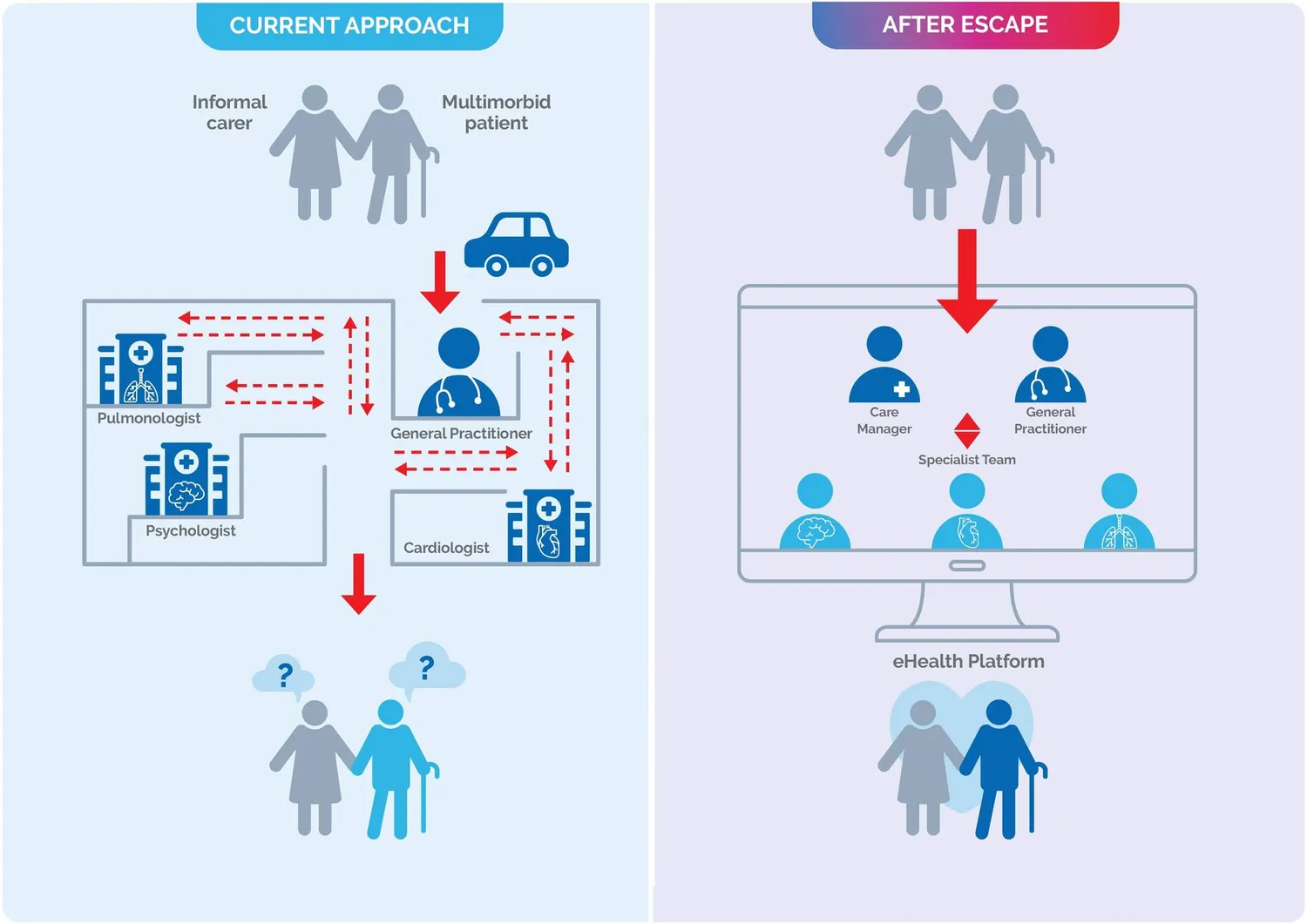
Using a cohort study with an embedded randomised control study, ESCAPE will assess the difference in health-related quality of life using our integrated care model versus traditional care methods. The participants in the study will be elderly, with chronic heart failure, at least two other chronic diseases, and coexisting mental disorder(s) or psychosocial distress.

Work Packages
Lead Partner: Syddansk Universitet (University of Southern Denmark)
Work Package Leader: Professor, Head of Department Susanne S. Pedersen
Contact: sspedersen@health.sdu.dk
Objectives
- Ensure the successful execution of ESCAPE by developing a comprehensive administrative and financial management plan
- Efficiently manage and coordinate the ESCAPE partners to ensure the work package objectives are met
- Guarantee high-quality project reporting to the European Commission
Lead Partner: Universitaetsklinikum Hamburg-Eppendorf (University Medical Centre Hamburg-Eppendorf)
Work Package Leader: Dr. Birgit Herbeck and Dr. Dagmar Lühmann
Contact: birgit.herbeck@med.uni-goettingen.de or d.luehmann@uke.de
Objectives
- Develop the blended-collaborative care (BCC) intervention, taking into consideration patient and carer preferences and variations within European healthcare systems, while utilising an electronic care plan manager
- Use machine learning to combine the BCC approach with multimorbidity data to ultimately create patient-centred treatment plans
September 2022 Update
In order to implement ESCAPE’s BCC intervention, there were a number of preliminary steps needed to ensure the intervention could be effectively and efficiently executed. This has been the primary focus of WP2 for the first eighteen months of the project.
First, a qualitative interview study was conducted in Denmark, Italy, and Germany to determine patients' and informal carers' needs and preferences related to the ESCAPE intervention. The results yielded eight “patient personas” – these personas were used to inform the intervention’s implementation, ensuring the process most closely reflected the needs of the patients (and carers).
Second, the ESCAPE Care Management Manual, an extensive document containing background information on multimorbidity and its consequences for patients, the principles of BCC and the intervention’s structure was developed. The manual serves as a comprehensive introduction to ESCAPE’s principles of care management for care managers, their trainers, and supervisors.
Third, was the tailoring and testing of imergo© - ESCAPE’s eHealth platform in collaboration with WP3. Addressing patients with multiple conditions requires a complex information system - to support this complexity, the imergo© platform was expanded.
And finally, because BCC and the care manager role is largely new to those implementing the intervention, training the team was essential - this was accomplished using the “train-the-trainer” technique.
Lead Partner: Fraunhofer Gesellschaft zur Foerderung der Angewandten Forschung E.V. (Fraunhofer Society for the Promotion of Applied Research e.V.)
Work Package Leader: Dr. Carlos A Velasco
Contact: carlos.velasco@fit.fraunhofer.de
Objectives
- Development of a scalable and flexible e-Health platform, based on the imergo® eHealth Integrated Care Platform (ICP), to allow for the coordination of different healthcare services and clinical providers
Lead Partner: Universitätsmedizin Göttingen (University Medical Centre Göttingen)
Work Package Leader: Professor Christoph Herrmann-Lingen
Contact: cherrma@gwdg.de
Objectives:
- Establish a study cohort of elderly patients from six EU countries with chronic heart failure, two or more medical comorbidities, and coexisting mental disorder or psychosocial distress who will be followed for ≥ 18 months
- Conduct a randomised controlled study embedded in the study cohort, to test the effectiveness of the blended-collaborative care approach on improving health-related quality of life and several secondary outcome measures
September 2022 Update:
The feasibility study, which tested the intervention arm of the main trial, was completed in June 2022 at the Göttingen site. The feasibility study included 10 patients who received the blended-collaborative care intervention for three months. The feasibility study provided the team with invaluable lessons on the screening and consenting process for patients, the assessment of the patient data from questionnaires and phone conversations, and the use of the patient registry. These learnings were then applied to the main clinical study to optimise the imergo® e-health platform and develop standard operation procedures (SOPs) and care managers guidelines.
Alongside the feasibility study, was the preparation of the main clinical trial. This involved the creation and translation of all study documents, that were then submitted to nine different ethical committees for approval. This laborious evaluation process took several months with back-and-forth communication to ensure compliance with all relevant authorities.
The official start of the clinical trial on April 1st was a big milestone and we were glad to achieve it on time. Recruitment first began at Göttingen followed by eight additional sites, Cologne, Hamburg, Leipzig, Roskilde, Slagelse, Kaunas, Bologna and Odense. As of September 29th, Göttingen has included 12 patients in the RCT and began the intervention. The other “green light” sites are currently screening patients for the RCT and will conduct their first baseline visits and randomisations in Fall 2022.
Lead Partner: Universitätsmedizin Göttingen (University Medical Centre Göttingen)
Work Package Leader: Tim Friede
Contact: tim.friede@med.uni-goettingen.de
Objectives
- Conduct statistical analyses on the demographic and clinical data from the WP4 clinical study
- Assess the cost-effectiveness of a holistic, patient-centred intervention, using the blended collaborative care approach
September 2022 Update
In WP5 “Evaluation of the effectiveness and cost-effectiveness of the intervention”, the statistical analyses of demographic, socioeconomic and clinical data (collected in the clinical study in WP4) will be conducted to evaluate the blended collaborative care (BCC) approach. The Department of Medical Statistics at the University Medical Centre Göttingen is responsible for the statistical aspects of the clinical trial and the statistical analyses of clinical data. The Institute of Health Economics and Clinical Epidemiology at the University of Cologne performs the cost-effectiveness evaluation.
The Health Economics team will examine the economic impact of BCC implementation from a provider perspective, taking into account participating countries. A health economic analysis ensures that the resources available in the healthcare system are used as efficiently as possible. This enables the greatest possible benefit for patients regarding the treatments used in their care, given possible cost constrains. WP5 is now focused on finalizing all materials that are necessary to collect country-specific cost and pricing information.
The Department of Medical Statistics took responsibility for the statistical aspects of the clinical trial design and will perform the statistical analyses necessary to evaluate the clinical effectiveness of the BCC approach. To improve reproducibility, transparency, and validity among clinical trials a Statistical Analysis Plan (SAP) is necessary. Our team of biostatisticians are currently preparing the SAP, which prespecifies and describes statistical methods for the analysis of the trial data. Furthermore, the team has contributed to the publication of the study protocol, already outlining the planned analyses.
Lead Partner: Syddansk Universitet (University of Southern Denmark)
Work Package Leader: Jakob Ousager
Contact: jousager@sdu.dk
Objectives
- Ensure that ethical standards and GDPR regulations are maintained and applied throughout the project
Lead Partner: ERINN Innovation
Work Package Leader: Rochelle Caruso
Contact: rochelle@erinn.eu
Objectives
- To develop and implement an effective dissemination and communication strategy for the project that can raise awareness of the project, its activities, and results to major stakeholder groups
